Published On Sep 2, 2021
شرح درس
Atomic emission spectra
CHEMISTRY - Secondary 2
In This part we will study:
Atomic emission spectra
• On heating atoms of a pure element - in gaseous or vapor state - to high temperatures or exposing them to a low pressure inside an electrical discharge tube, they emit a radiation called emission spectrum (line spectrum).
• On examining this radiant light by a device called spectroscope, it was found that it is composed of a limited number of restricted colored lines separated by dark areas. So, it is called line spectrum.
• It is worth mentioning that the physicists - at that time - were not able to explain this phenomenon.
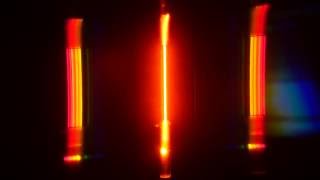
The line spectrum of hydrogen atom.
• The line spectrum of hydrogen atom appears (on examining) as four colored lines separated by dark areas, as in the following figure:
The line spectrum is an essential characteristic for each element.
Because there are no two elements have the same spectral lines.
Bohr's atomic model (1913)
The study of atomic spectra is considered the key which solved the puzzle of the atomic structure. That was achieved by the Danish scientist Niels Bohr upon which he was rewarded by the Nobel Prize in 1922
Bohr's postulates
A - Points that agree with Rutherford's postulates
A positively charged nucleus exists in the center of the atom.
The number of negative electrons (revolving around the nucleus) equals the number of positive protons inside the nucleus.
During the revolving of the electron around the nucleus, a centrifugal force arises which is equivalent to the attraction force of the nucleus on the electron.
B - New postulates
Electrons orbit the nucleus in a rapid movement without emission or absorption of any amount of energy and the atom in this case named stable atom.
Electrons orbit the nucleus in definite allowed energy levels. They can't be found at intermediate distances, where the electron moves from an energy level to another one via a complete jumping.
Each electron in the atom has a definite amount of energy depending on the distance between its energy level and the nucleus, the energy of any level increases as its radius increases. Each energy level is expressed by an integer number called the principal quantum number (n), and the electron revolves in the lowest allowed energy level in its ground state.
When the electron acquires a quantity of energy - known as quantum - by heating or by electric discharge, the electron jumps temporarily to a higher energy level.
• This is in case that the absorbed quantum of energy is equal to the difference in energies between the two levels, and the atom in this case is known as excited atom
• Since the electron in the excited atom is unstable, it returns back to its original level with emission of the same quantum of energy (photon) in the form of radiant light that appears in the form of a characteristic spectral line of a certain wavelength and frequency in addition to other invisible lines.
The acquired amount of energy (the quantum) when an electron transfers from its ground state to the excited state equals the amount of energy which is released when this electron returns back to its ground state level.
The multitude of atoms absorb different amounts of energy, then radiate their energies producing spectral lines. These spectral lines indicate the energy levels from which their electrons are transmitted back to the ground state.
Notes
The quantum of energy required to transfer an electron between the different energy levels is not equal.
Because the distance and the difference in energy between them are not equal.
The quantum of energy required to transfer an electron from an energy level to another decreases as we go further from the nucleus.
Because the energy gap decreases, as we go further from the nucleus.
The advantages and inadequacies of Bohr's atomic model
Best wishes to you
Mr.Ahmed Elbasha
كيمياء الصف الثاني الثانوي لغات - الفصل الدراسي الاول
شرح كيمياء لغات تانية ثانوي - مستر احمد الباشا